Rapid relief.
Lasting remission.
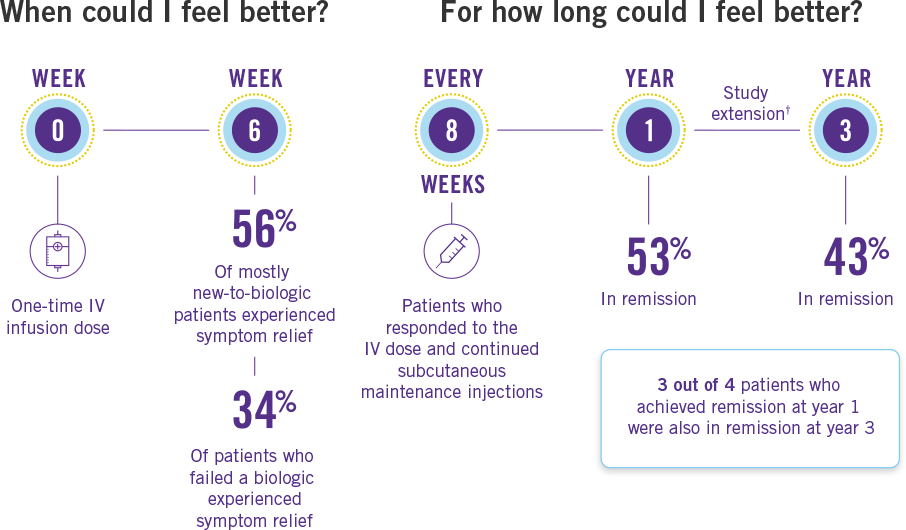
Clinical trials included patients who failed or were intolerant to other medications, including a biologic, prior to STELARA®. After only one IV dose of STELARA®, the majority of patients who were new to or had not failed a biologic found rapid relief from their Crohn’s disease symptoms in just 6 weeks, with noticeable improvement as early as 3 weeks* for some. Additionally, the majority of patients were in remission at 1 year after responding to the induction dose and continuing treatment with STELARA®.
*Symptom improvement was measured differently at weeks 3 and 6.
†After 1 year, patients and healthcare providers knew that STELARA® was being used. This may have increased results.
Individual results may vary. STELARA® is not for everyone. Only you and your doctor can decide if STELARA® is right for you.
The legacy of STELARA®

19+ YEARS
of combined clinical research
and 10+ years on the market
across indications

425,000+ PATIENTS
are estimated to have been treated with STELARA® for Crohn’s disease and other indications in the US†

100,000+ PATIENTS
are estimated to have been treated with STELARA® for Crohn’s disease and ulcerative colitis in the US‡
†This is based on information licensed from IQVIA™: LAAD claims, NPA, DDD for the period 2009-2020 reflecting estimates of real-world activity and based on custom methodologies developed by Janssen. All rights reserved.
‡Estimations are based on total STELARA® distributed for CD (91,000) and UC (9,000) from October 2016 to July 2020.
What is STELARA® and how does it work?
- STELARA® is a prescription biologic medicine
- Many biologics are made from proteins, genes, or antibodies
- Some biologics target enzymes or proteins that may cause inflammation—like the inflammation thought to cause Crohn’s disease symptoms
STELARA® works differently
There are many different naturally occurring proteins in the body that contribute to inflammation. Patients with Crohn’s disease are found to have elevated levels of two of these proteins, IL-12 and IL-23.
STELARA® is the only FDA-approved medicine that targets IL-12 and IL-23, which are thought to be associated with gastrointestinal inflammation in Crohn’s disease.
Learn about dosing with STELARA®
Start with a
one-time IV infusion
STELARA® treatment starts with a one-time intravenous (IV) infusion through a vein in your arm that provides the amount of medication based on your body weight and is administered by a medical professional in a comfortable setting. It takes at least 1 hour to receive the full dose of medicine.
Keep going with
maintenance injections
After the one-time IV infusion, you will receive STELARA® as an injection under the skin (subcutaneous injection) every 8 weeks. There are 6 injections during the first year of treatment. Your doctor can administer at his or her office, or your doctor will decide if you or a caregiver may give your injections of STELARA® at home.
STELARA® is intended for use under the guidance and supervision of your doctor. If your doctor decides that you or a caregiver may give your injections of STELARA® at home, you should receive training on the right way to prepare and inject STELARA®.
The STELARA® dosing difference
First 52 weeks of therapy after starter doses
STELARA®
(ustekinumab)
6
INJECTIONS
ONE 90 MG INJECTION
EVERY 8 WEEKS
After 1 starter IV infusion, which should take at least one hour.
HUMIRA®§
(adalimumab)
25
INJECTIONS
ONE 40 MG INJECTION
EVERY OTHER WEEK
After 2 injections of 80 mg on day 1, then 1 injection of 80 mg on day 15. Dosing with citrate-free HUMIRA®.
STELARA®
(ustekinumab)
STELARA® is a prescription medicine used to treat adults 18 years and older with moderately to severely active Crohn's disease who have already taken other medicine that did not work well enough or they could not tolerate it.
HUMIRA®§
(adalimumab)
HUMIRA® is a prescription medicine used to treat moderate to severe Crohn’s disease (CD) in adults.
While these factors are important, there are additional considerations for selecting treatment. Please talk to your doctor about treatment options and what might be right for you.
This presentation is not intended to compare the safety and effectiveness of these treatments. Please refer to each product’s full Prescribing Information for recommended dosing and administration.
§Indicated trademark is registered trademark of its respective owner.
STELARA® Safety
Ask your doctor about the benefits and risks of STELARA®. Prescription medications, including STELARA®, have possible risks involved with treatment, so it’s important to discuss them with your doctor.
STELARA® is not for everyone; only your doctor can decide if it's right for you. STELARA® is a prescription medicine that affects your immune system. It can increase your chance of having serious side effects including serious infections, cancer, serious allergic reactions, lung inflammation, and a rare condition called posterior reversible encephalopathy syndrome.
These are not all the possible side effects of STELARA®.
Please read the Important Safety Information located at the bottom of the screen and the Medication Guide for STELARA® to learn more about these and other risks for STELARA®. Discuss any questions you have with your doctor.
In clinical studies, the most common side effects were:
- nasal congestion, sore throat, and runny nose
- upper respiratory infections
- fever
- headache
- tiredness
- itching
- nausea and vomiting
- redness at the injection site
- vaginal yeast infections
- urinary tract infections
- sinus infection
- bronchitis
- diarrhea
- stomach pain
- joint pain
These are not all of the possible side effects of STELARA®. Call your doctor for medical advice about side effects.
You should not receive a live vaccine while taking STELARA®. Before receiving STELARA®, tell your doctor if you have recently received or are scheduled to receive an immunization (vaccine). Tell your doctor if anyone in your house needs a vaccine. The viruses used in some types of vaccines can spread to people with a weakened immune system, and can cause serious problems. You should not receive the BCG vaccine during the one year before taking STELARA® or one year after you stop taking STELARA®.
Is STELARA® right for you?
Ask your doctor today.
When it comes to getting control of your Crohn’s disease symptoms, you are your own best advocate. The road to remission starts with an open and honest conversation with your doctor.
The importance of a positive doctor-patient relationship

" Your relationship with your doctor, your ability to communicate with one another, is as vital as your treatment plan itself.
STELARA® patient brochure
If you want to learn more about STELARA®, please download our patient brochure.
RAPID RELIEF
The majority of patients treated with STELARA® in a clinical study who were new to or had not failed a biologic saw relief from their Crohn’s disease symptoms in just 6 weeks
LASTING REMISSION
The majority of patients were in remission at 1 year after responding to the one-time IV infusion dose and continuing on STELARA® treatment
Individual results may vary. STELARA® is not for everyone. Only you and your doctor can decide if STELARA® is right for you.
Once you and your doctor have decided STELARA® is right for you, STELARA withMe will help you find the resources you may need to get started and stay on track. We will give you information on your insurance coverage, potential out-of-pocket costs, and treatment support, and identify options that may help make your treatment more affordable.
Support for Commercially Insured Patients
The STELARA withMe Savings Program
If you have commercial insurance
- Eligible patients pay $5 per dose
- Maximum program benefit per calendar year shall apply
- Terms expire at the end of each calendar year and may change
- Program does not cover the cost to give you your treatment
- See full program requirements at STELARAwithMeSavings.com
Click to enroll in STELARA withMe